NASA is planning to land a crew on the Moon by 2024, and then onward to Mars, possibly in the 2030s. One day, we will have permanently crewed bases on both worlds. Unlike the initial short-stay visits, long-term bases will have to be self sufficient in as many essentials as possible.
A lot of research has gone into preparing for In Situ Resource Utilization (ISRU) that could help to build and sustain a lunar base. Now, similar ideas for Mars are catching up, with a new study, published in PNAS, suggesting a way to use the brine (salty water) found on Mars to make breathable air and fuel.
“Living off the land” will be even more important on Mars than on the Moon, because Mars is much further away – making transport costs (and time) correspondingly greater.
One major resource issue is how to provide enough oxygen for the Mars-base crew to breathe. Mars has only a thin atmosphere, with a surface pressure less than a hundredth of the Earth’s. Even worse, it is 96% carbon dioxide with only about 0.1% oxygen. Earth’s atmosphere is 21% oxygen.
NASA’s Mars2020 rover, Perseverance, which is already on its way to Mars, carries an experiment called MOXIE, a name imaginatively contrived from Mars OXygen In situ Experiment.
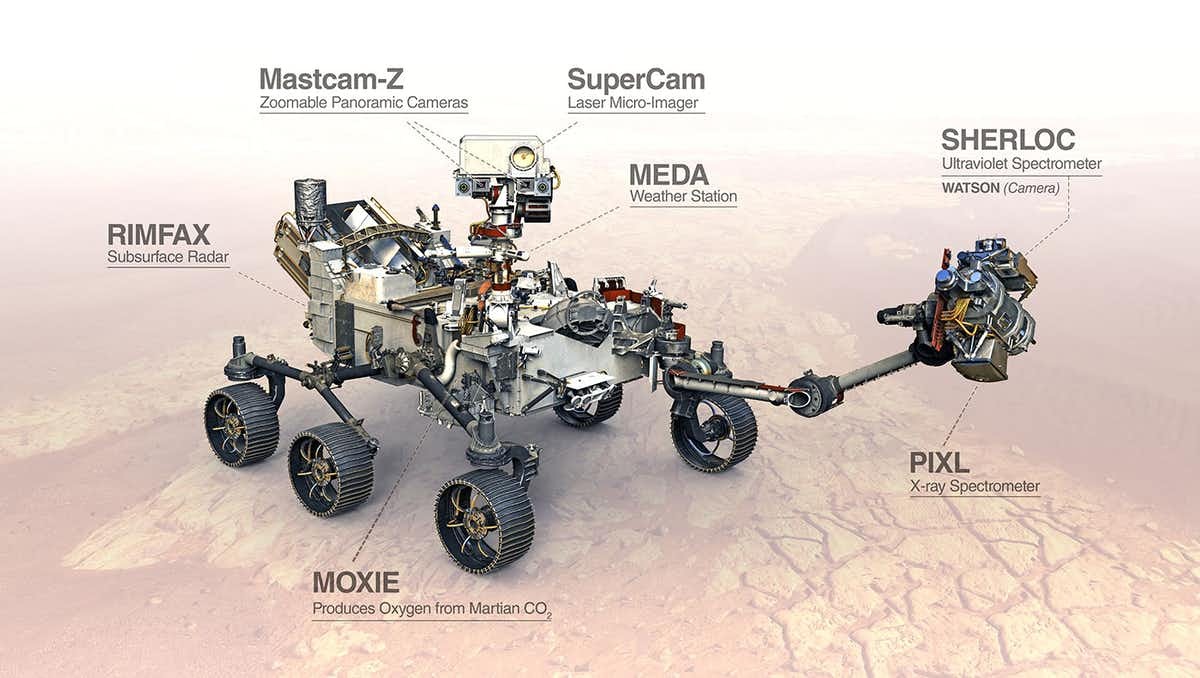
MOXIE’s purpose is to demonstrate that oxygen can be made from the carbon dioxide in Mars’s atmosphere by using electricity to split it into a mixture of oxygen and carbon monoxide, via a process called electrolysis. If this works as expected, the oxygen could be collected and used to give colonists something to breathe or as a component in fuel. The carbon monoxide would be unwanted, and would be vented back into the martian atmosphere.
Oxygen from martian brine
A new way has emerged, however, that would consume 25 times less electrical power to produce the same amount of oxygen. No matter whether you use solar cells or a radioactive source to generate your electricity, the available power is limited, so this is an important gain.
In the new study, a team from Washington University in the US, demonstrate how electrolysis can be used efficiently to produce oxygen and hydrogen simultaneously from brine. It turns out that when you start from a concentrated solution of magnesium perchlorate, it is relatively easy to split the water component of the brine into oxygen and hydrogen using electrolysis.
This may sound exotic, but magnesium perchlorate is what the briny water at and near the surface of Mars appears to consist of, as seen for example when liquid droplets appeared on the legs of NASA’s Phoenix lander, which touched down in Mars’s far north in 2008. The Curiosity rover has also found evidence of calcium perchlorate brine just south of the martian equator.
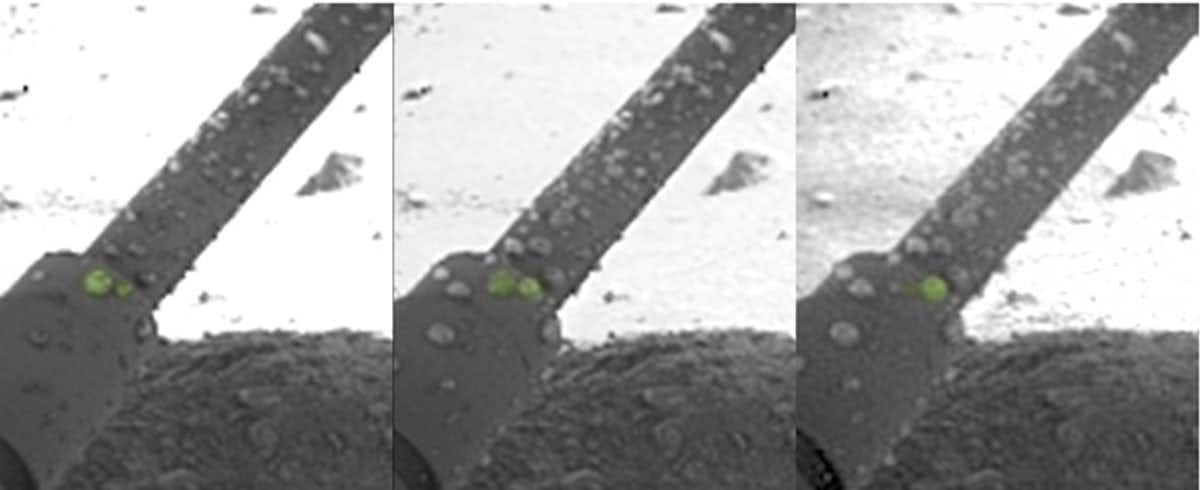
Perchlorate salts are good at scavenging water from the driest of atmospheres, hence the droplets on the Phoenix lander’s legs. They can depress the freezing point of a liquid to as low as −70 °C, which prevents concentrated perchlorate brines from freezing even at Mars’s low surface temperatures. There are places where the appearance of dark, moist streaks is thought to be the seasonal flow of brine to the surface.
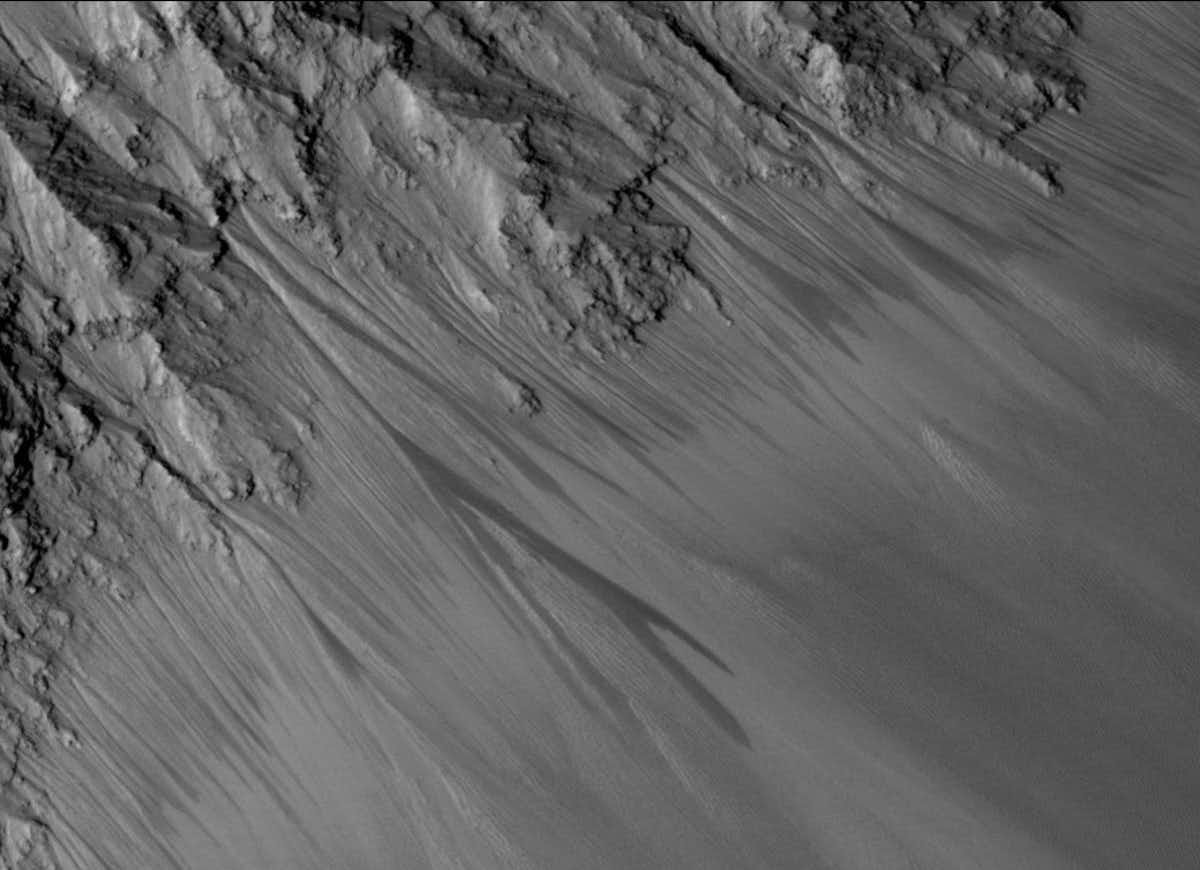
If you land where there is brine available, the new study argues, you could make as much oxygen as you like – provided you have unlimited brine and power. The breakthrough in the efficiency of this perchlorate brine electrolysis has to do with the make-up of the oxygen-producing electrode. For this, the study used a variety of a mineral called pyrochlore, consisting in this instance of an oxide of lead and ruthenium. Pyrochlores have a wide range of technological applications, including, as in this case, as an “electrocatalyst” to make electrolysis faster and easier.
Practical options
It remains to be seen whether MOXIE-style electrolysis of martian carbon dioxide or pyrochlore-enabled electrolysis of martian brines proves the more practical way to make oxygen on Mars. The hydrogen from brine electrolysis is a bonus that you don’t get by electrolysis of carbon dioxide, and this could be used as rocket fuel as the study points out. Actually, if you want to do that, you’d need to use up the oxygen as the complimentary component of the fuel. But at least that gives you a choice: breathe the oxygen or use it in a hydrogen-plus-oxygen fuel mixture.
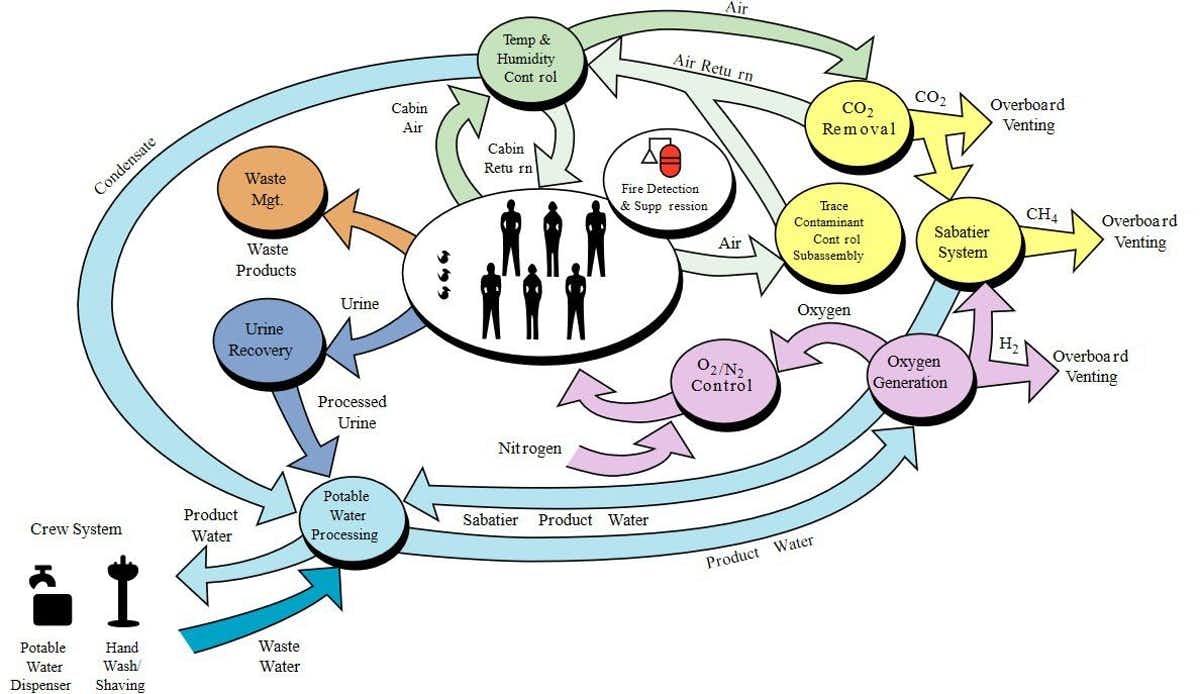
Neither option would be available during the several month long journey to and from Mars, for which recycling solutions would have to be found, as today on the International Space Station. These would also be important on the surface of Mars.
There is another way to replenish oxygen of course, which would be to grow plants in the Mars base. These could absorb the carbon dioxide exhaled by the crew and liberate oxygen by photosynthesis. Crew members could also eat some of the plants, which would be a welcome source of fresh food.