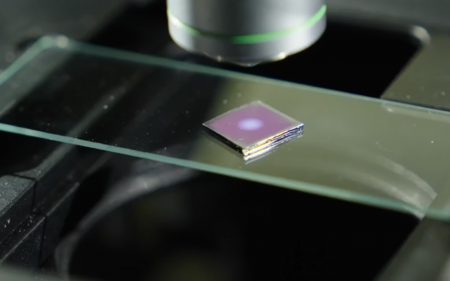
Life on Earth owes its existence to photosynthesis – a process which is 2.3 billion years old. This immensely fascinating (and still not fully understood) reaction enables plants and other organisms to harvest sunlight, water and carbon dioxide while converting them into oxygen and energy in the form of sugar.
Photosynthesis is such an integral part of Earth’s functioning that we pretty much take it for granted. But as we look beyond our own planet for places to explore and settle on, it is obvious how rare and valuable the process is.
As my colleagues and I have investigated in a new paper, published in Nature Communications, recent advances in making artificial photosynthesis may well be key to surviving and thriving away from Earth.
The human need for oxygen makes space travel tricky. Fuel constraints limit the amount of oxygen we can carry with us, particularly if we want to do long-haul journeys to the Moon and Mars. A one-way trip to Mars usually takes on the order of two years, meaning we can’t easily send supplies of resources from Earth.
There are already ways to produce oxygen by recycling carbon dioxide on the International Space Station. Most of the ISS’s oxygen comes from a process called “electrolysis”, which uses electricity from the station’s solar panels to split water into hydrogen gas and oxygen gas, which astronauts breathe in. It also has a separate system converting the carbon dioxide the astronauts breathe out into water and methane.
But these technologies are unreliable, inefficient, heavy and difficult to maintain. The oxygen generation process, for example, requires about one third of the total energy needed to run ISS’s entire system supporting “environmental control and life support”.
Ways forward
The search for alternative systems which can be employed on the Moon and on trips to Mars is therefore ongoing. One possibility is to harvest solar energy (which is abundant in space) and directly use it for oxygen production and carbon dioxide recycling in only one device.
The only other input in such a device would be water – similar to the photosynthesis process going on in nature. That would circumvent complex set-ups where the two processes of light harvesting and chemical production are separated, such as on the ISS.
This is interesting as it could reduce the weight and volume of the system – two key criteria for space exploration. But it would also be more efficient.
We could use additional thermal (heat) energy released during the process of capturing solar energy directly for catalysing (igniting) the chemical reactions – thereby speeding them up. Moreover, complex wiring and maintenance could be significantly reduced.
We produced a theoretical framework to analyse and predict the performance of such integrated “artificial photosynthesis” devices for applications on Moon and Mars.
Instead of chlorophyll, which is responsible for light absorption in plants and algae, these devices use semiconductor materials which can be coated directly with simple metallic catalysts supporting the desired chemical reaction.
Our analysis shows that these devices would indeed be viable to complement existing life support technologies, such as the oxygen generator assembly employed on the ISS. This is particularly the case when combined with devices which concentrate solar energy in order to power the reactions (essentially large mirrors which focus the incoming sunlight).
There are other approaches too. For example, we can produce oxygen directly from lunar soil(regolith). But this requires high temperatures to work.
Artificial photosynthesis devices, on the other hand, could operate at room temperature at pressures found on Mars and the Moon. That means they could be used directly in habitats and using water as the main resource.
This is particularly interesting given the stipulated presence of ice water in the lunar Shackleton crater, which is an anticipated landing site in future lunar missions.
Read More: Satellites and space junk may make dark night skies brighter, hindering astronomy and hiding stars from our view
On Mars, the atmosphere composes of nearly 96% carbon dioxide – seemingly ideal for an artificial photosynthesis device. But the light intensity on the red planet is weaker than on Earth due to the larger distance from the Sun.
So would this pose a problem? We actually calculated the sunlight intensity available on Mars. We showed that we can indeed use these devices there, although solar mirrors become even more important.
The efficient and reliable production of oxygen and other chemicals as well as the recycling of carbon dioxide onboard spacecrafts and in habitats is a tremendous challenge that we need to master for long-term space missions.
Existing electrolysis systems, operating at high temperatures, require a significant amount of energy input. And devices for converting carbon dioxide to oxygen on Mars are still in their infancy, whether they are based on photosynthesis or not.
So several years of intense research are necessary to be able to use this technology in space. Copying the essential bits from nature’s photosynthesis could give us some advantages, helping us realising them in the not-too-distant future.
Use in space and on Earth
The returns would be huge. For example, we could actually create artificial atmospheres in space and produce chemicals we require on long-term missions, such as fertilisers, polymers or pharmaceuticals.
Additionally, the insights we gain from designing and fabricating these devices could help us meet the green energy challenge on Earth.
We are fortunate enough to have plants and algae for producing oxygen. But artificial photosynthesis devices could be used to produce hydrogen or carbon-based fuels (instead of sugars), opening up a green way for the production of energy-rich chemicals which we can store and use in transport.
The exploration of space and our future energy economy have a very similar long-term goal: sustainability. Artificial photosynthesis devices may well become a key part of realising it.
- is an Assistant Professor in Catalysis, University of Warwick
- This article first appeared on The Conversation