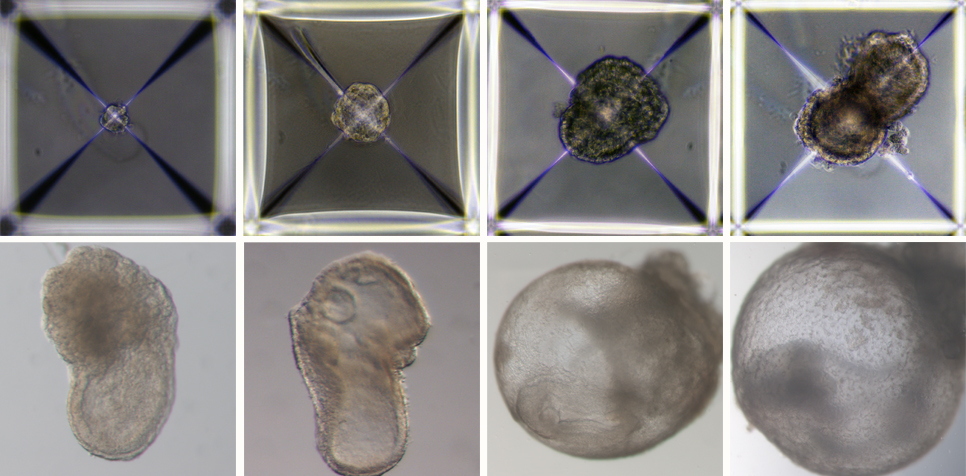
In what’s reported as a world-first achievement, biologists have grown mouse embryo models in the lab without the need for fertilised eggs, embryos, or even a mouse – using only stem cells and a special incubator.
This achievement, published in the journal Cell by a team led by researchers from the Weizmann Institute of Science in Israel, is a very sophisticated model of what happens during early mouse embryo development – in the stage just after implantation.
This is a crucial stage: in humans, many pregnancies are lost around this stage, and we don’t really know why. Having models provides a way to better understand what can go wrong, and possibly insights into what we may be able to do about it.
The tiniest cluster
What’s particularly interesting about the newly published model is its very complex structure; not only does it mimic the cell specification and layout of an early-stage body plan – including precursors of heart, blood, brain and other organs – but also the “support” cells like those found in the placenta and other tissues required to establish and maintain a pregnancy.
The earliest stages of pregnancy are difficult to study in most animals. The embryos are microscopic, tiny clusters of cells, difficult to locate and observe within the uterus.
But we do know that at this stage of development, things can go awry; for example, environmental factors can influence and interfere with development, or cells fail to receive the right signals to fully form the spinal cord, such as in spina bifida. Using models like this, we can start to ask why.
However, even though these models are a powerful research tool, it is important to understand they are not embryos.
They replicate only some aspects of development, but not fully reproduce the cellular architecture and developmental potential of embryos derived after fertilisation of eggs by sperm – so-called natural embryos.
The team behind this work emphasises they were unable to develop these models beyond eight days, while a normal mouse pregnancy is 20 days long.
Are ‘synthetic embryos’ of humans on the horizon?
The field of embryo modelling is progressing rapidly, with new advances emerging every year.
In 2021, several teams managed to get human pluripotent stem cells (cells that can turn into any other type of cell) to self-aggregate in a Petri dish, mimicking the “blastocyst”. This is the earliest stage of embryonic development just before the complex process of implantation, when a mass of cells attach to the wall of the uterus.
Researchers using these human embryo models, often called blastoids, have even been able to start to explore implantation in a dish, but this process is much more challenging in humans than it is in mice.
Growing human embryo models of the same complexity that has now been achieved with a mouse model remains a distant proposition, but one we should still consider.
Importantly, we need to be aware of how representative such a model would be; a so-called synthetic embryo in a Petri dish will have its limitations on what it can teach us about human development, and we need to be conscious of that.
Ethical pitfalls
No embryonic modelling can happen without a source of stem cells, so when it comes to thinking about the future use of this technology, it is vital to ask – where are these cells coming from? Are they human embryonic stem cells (derived from a blastocyst), or are they induced pluripotent stem cells? The latter can be made in the lab from skin, or blood cells, for example, or even derived from frozen samples.
An important consideration is whether using cells for this particular type of research – trying to mimic an embryo in a dish – requires any specific consent. We should be thinking more about how this area of research will be governed, when should it be used, and by whom.
However, it is important to recognise that there are existing laws and international stem cell research guidelines that provide a framework to regulate this area of research.
In Australia, research involving human stem cell embryo models would require licensing, similar to that required for the use of natural human embryos under law that has been in place since 2002. However, unlike other jurisdictions, Australian law also dictates how long researchers can grow human embryo models, a restriction that some researchers would like to see changed.
Regardless of these or other changes to how and when human embryo research is conducted, there needs to be greater community discourse around this subject before a decision is made.
There is a distinction between banning the use of this technology and technologies like cloning in humans for reproductive use, and allowing research using embryo models to advance our understanding of human development and developmental disorders that we can’t answer by any other means.
The science is rapidly advancing. While mostly in mice at this stage, now is the time to discuss what this means for humans, and consider where and how we draw the line in the sand as the science evolves.
- is Professor Emerging Technologies (Stem Cells), The University of Melbourne
- This article first appeared on The Conversation